Having an extra electron crossword – Embark on an enlightening journey into the captivating world of anions, where the presence of an extra electron orchestrates a symphony of chemical intrigue. Dive into the fascinating realm of anion formation, electron affinity, and the intricate dance of valence electron configurations.
Prepare to unravel the secrets of size and charge effects, unravel the complexities of polyatomic anions, and witness the remarkable applications of these enigmatic species in various fields.
Unveiling the mysteries of anions, we will explore their fundamental characteristics, delve into their formation processes, and unravel their unique properties. From the stability of anion configurations to the impact of size and charge, no stone will be left unturned in our quest for knowledge.
Anion Formation and Electron Affinity: Having An Extra Electron Crossword
Anions are negatively charged ions formed when an atom or molecule gains one or more electrons. The process of anion formation is influenced by the electron affinity of the atom or molecule, which is the energy change that occurs when an electron is added to a neutral atom or molecule.
Elements or ions that readily form anions typically have low electron affinities. This means that it is relatively easy for them to accept an electron and become negatively charged. For example, halogens (Group 17 elements) have high electron affinities and readily form anions.
Fluorine, the most electronegative element, has the highest electron affinity (328 kJ/mol) and readily forms the fluoride anion (F –).
, Having an extra electron crossword
The electron affinity of an atom or ion can vary depending on its size, charge, and electronic configuration. Larger atoms and ions generally have lower electron affinities than smaller ones. This is because the electrons in larger atoms and ions are farther from the nucleus and experience less electrostatic attraction.
Positively charged ions have lower electron affinities than neutral atoms, while negatively charged ions have higher electron affinities. This is because the electrostatic repulsion between the electrons in a negatively charged ion makes it more difficult to add another electron.
Valence Electron Configuration
The valence electron configuration of an element plays a crucial role in determining the stability of its anion. Anions are formed when an atom gains one or more electrons, resulting in a negative charge. The stability of an anion depends on the number and arrangement of its valence electrons.
Elements with stable anion configurations tend to have a high affinity for electrons, which means they readily accept electrons to form anions. These elements typically have valence electron configurations that are close to a noble gas configuration, which is characterized by a full outer electron shell.
Noble Gas Configuration
Noble gases are the most stable elements because they have a full outer electron shell, which gives them a low chemical reactivity. Anions formed by elements that have valence electron configurations similar to noble gases are also stable because they have a similar electron configuration to the noble gas.
For example, chlorine has a valence electron configuration of 3s 23p 5, which is one electron short of a noble gas configuration (3s 23p 6). When chlorine gains one electron, it forms the chloride anion (Cl –), which has a valence electron configuration of 3s 23p 6, which is the same as the noble gas argon.
Other elements that form stable anions include oxygen, nitrogen, and fluorine. These elements all have valence electron configurations that are close to a noble gas configuration, and they readily accept electrons to form anions.
Size and Charge Effects
The size and charge of an anion significantly impact its stability and behavior in chemical reactions. These factors influence the anion’s electrostatic interactions with other ions and molecules, affecting its ability to form stable compounds and its overall reactivity.
Size of Anions
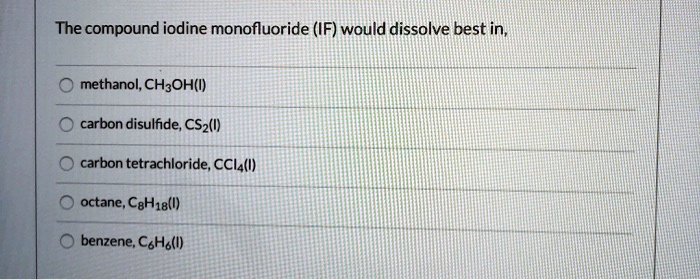
Larger anions have a more diffuse electron cloud, resulting in weaker electrostatic interactions. This makes them less reactive and more stable. For example, the iodide ion (I –) is larger than the fluoride ion (F –) and, therefore, less reactive and more stable.
Charge of Anions
Anions with a higher charge have a stronger electrostatic attraction for cations, leading to increased reactivity and instability. For example, the sulfate ion (SO 42-) has a higher charge than the chloride ion (Cl –) and is, therefore, more reactive and less stable.
Polyatomic Anions
Polyatomic anions are ionic species composed of multiple atoms that bear a negative charge. They form when a group of atoms gains one or more electrons, resulting in an overall negative charge on the molecule.
Common Polyatomic Anions
Some of the most common polyatomic anions include:
- Hydroxide (OH –): Consists of an oxygen atom bonded to a hydrogen atom with a lone pair of electrons on the oxygen atom.
- Oxide (O 2-): Consists of two oxygen atoms bonded together with a double bond.
- Carbonate (CO 32-): Consists of a central carbon atom surrounded by three oxygen atoms arranged in a trigonal planar geometry.
- Sulfate (SO 42-): Consists of a central sulfur atom surrounded by four oxygen atoms arranged in a tetrahedral geometry.
- Nitrate (NO 3–): Consists of a central nitrogen atom surrounded by three oxygen atoms arranged in a trigonal planar geometry.
Structures and Bonding Characteristics
The structures and bonding characteristics of polyatomic anions vary depending on the specific atoms involved and the number of electrons gained. In general, polyatomic anions tend to have:
- Covalent bonds between the atoms within the anion.
- A net negative charge due to the presence of extra electrons.
- A specific geometry that is determined by the number and arrangement of the atoms and the electron pairs involved in bonding.
Applications of Anions
Anions play crucial roles in various fields, including chemistry, industry, and everyday life. Their unique properties make them essential components in a wide range of applications.
In the chemical industry, anions are utilized as intermediates in the production of numerous compounds, including acids, bases, and salts. They are also employed as catalysts in various chemical reactions, enhancing the efficiency and selectivity of these processes.
Batteries
Anions are vital components in batteries, where they participate in electrochemical reactions that generate electricity. In lead-acid batteries, for instance, the sulfate anion (SO 42-) acts as the electrolyte, facilitating the flow of ions between the electrodes during charging and discharging cycles.
Fertilizers
Anions are essential for plant growth and development. Nitrogen-containing anions, such as nitrate (NO 3–) and ammonium (NH 4+), are major components of fertilizers, providing nitrogen to plants for protein synthesis and other metabolic processes.
Other Applications
- Anions are used in the production of glass, ceramics, and other materials.
- They are employed as food additives, such as emulsifiers and stabilizers.
- Anions play a role in environmental processes, such as water purification and wastewater treatment.
FAQ Section
What is the significance of electron affinity in anion formation?
Electron affinity plays a crucial role in determining the ease of anion formation. Elements with high electron affinities readily accept electrons to form stable anions.
How does valence electron configuration influence anion stability?
Valence electron configuration dictates the stability of anions. Elements with stable electron configurations, such as noble gas configurations, form more stable anions.
What factors affect the size and charge of anions?
The size and charge of anions are influenced by the number of electrons and protons in the ion. Larger anions have more electrons, while anions with higher charges have fewer electrons.
Can you provide examples of polyatomic anions?
Common polyatomic anions include hydroxide (OH-), carbonate (CO32-), and sulfate (SO42-).
What are some practical applications of anions?
Anions find applications in various fields, including batteries, fertilizers, and water treatment.