Introducing the Neutralization Reactions Worksheet Answer Key, an invaluable resource for understanding the fundamentals of neutralization reactions. This guide provides a comprehensive overview of the topic, from its basic concepts to its diverse applications.
Neutralization reactions play a crucial role in various scientific disciplines and everyday life. They involve the interaction between acids and bases, resulting in the formation of salt and water. Balancing these reactions is essential for predicting the products and quantities involved.
1. Introduction
Neutralization reactions are chemical reactions that occur between an acid and a base, resulting in the formation of a salt and water. These reactions are important in various fields, including chemistry, biology, and environmental science.
2. Types of Neutralization Reactions
- Complete neutralization: Occurs when the acid and base react in stoichiometric proportions, completely consuming both reactants.
- Incomplete neutralization: Occurs when either the acid or base is in excess, resulting in a solution with a pH that is not neutral.
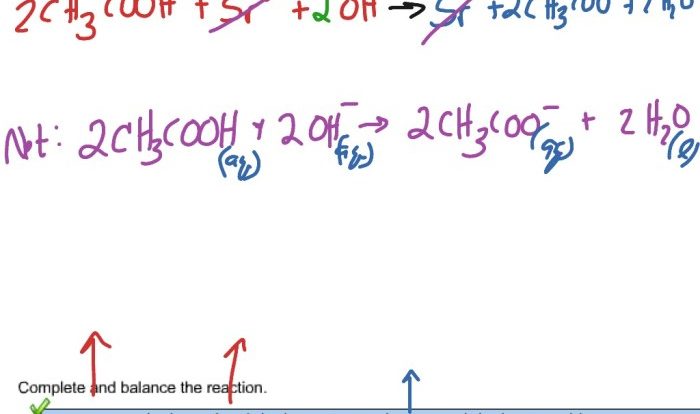
3. Balancing Neutralization Reactions
To balance a neutralization reaction, the following steps are taken:
- Write the unbalanced equation for the reaction.
- Balance the number of atoms of each element on both sides of the equation.
- Add coefficients to the reactants and products to balance the charges.
4. Applications of Neutralization Reactions: Neutralization Reactions Worksheet Answer Key

- Everyday life: Neutralization reactions are used in antacids, cleaning products, and water treatment.
- Industries: Neutralization reactions are used in the production of fertilizers, pharmaceuticals, and textiles.
- Environmental implications: Neutralization reactions are used to treat acidic wastewater and neutralize soil acidity.
Essential Questionnaire
What is the purpose of a neutralization reaction?
Neutralization reactions neutralize the acidic or basic properties of a solution, resulting in a neutral or slightly acidic/basic solution.
How do you balance a neutralization reaction?
To balance a neutralization reaction, adjust the coefficients of the reactants and products to ensure that the number of atoms of each element is equal on both sides of the equation.
What are the applications of neutralization reactions?
Neutralization reactions have numerous applications, including pH control in swimming pools, food preservation, and the production of fertilizers and pharmaceuticals.