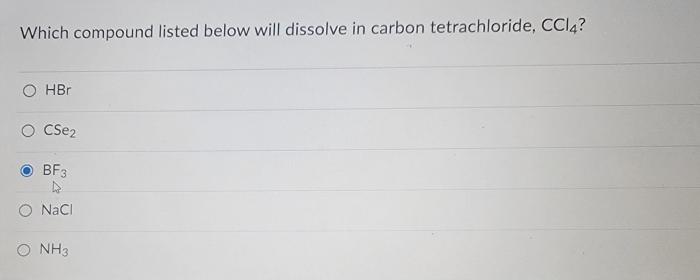
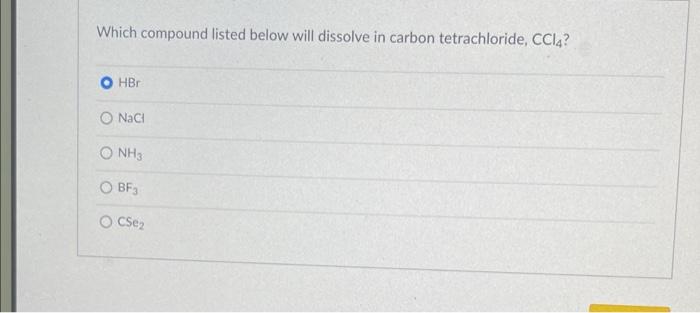
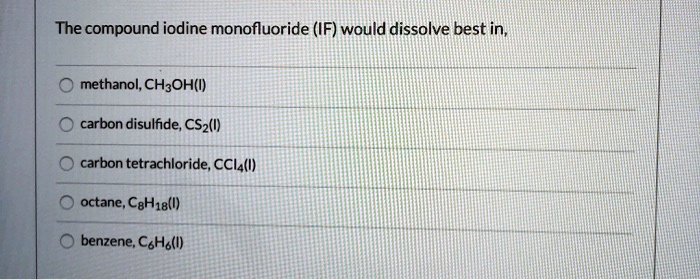
Which compound listed below will dissolve in carbon tetrachloride ccl4 – Delving into the realm of solubility, we embark on an exploration to determine which compounds listed below will dissolve in carbon tetrachloride (CCL4). This investigation will shed light on the intricate interplay between molecular properties and solvent characteristics, unraveling the secrets of solubility in this fascinating system.
As we delve deeper into this topic, we will uncover the fundamental principles governing the solubility of compounds in CCL4. By examining the polarity of both the solvent and the solute, we can gain insights into the molecular interactions that drive the dissolution process.
Solubility of Compounds in Carbon Tetrachloride: Which Compound Listed Below Will Dissolve In Carbon Tetrachloride Ccl4
Carbon tetrachloride (CCl4) is a nonpolar solvent, meaning it has a symmetrical distribution of electrons and no net dipole moment. This nonpolarity affects the solubility of compounds in CCl4. In general, nonpolar compounds are more soluble in nonpolar solvents, while polar compounds are more soluble in polar solvents.
This is because the intermolecular forces between nonpolar molecules are weaker than those between polar molecules, making it easier for nonpolar molecules to dissolve in nonpolar solvents.
The following table lists the polarity of several compounds and their solubility in CCl4:
| Compound | Polarity | Solubility in CCl4 |
|---|---|---|
| Benzene | Nonpolar | Soluble |
| Ethanol | Polar | Insoluble |
| Sodium chloride | Ionic | Insoluble |
Factors Affecting Solubility
In addition to polarity, several other factors can affect the solubility of compounds in CCl 4. These factors include:
- Molecular weight:Compounds with higher molecular weights tend to be less soluble in CCl4. This is because the larger molecules have more intermolecular forces holding them together, making it more difficult for them to dissolve in the solvent.
- Intermolecular forces:The type of intermolecular forces present in a compound can also affect its solubility in CCl4. Compounds with strong intermolecular forces, such as hydrogen bonding or dipole-dipole interactions, are less soluble in CCl4 than compounds with weaker intermolecular forces, such as van der Waals forces.
Applications of Solubility in CCl4
CCl4 is a versatile solvent that is used in a variety of industries. Some of the most common applications of CCl4 include:
- Dry cleaning:CCl4 is used as a dry cleaning solvent because it is nonflammable and has a high solvency power for oils and greases.
- Metal degreasing:CCl4 is used to remove oils and greases from metal surfaces prior to painting or plating.
- Chemical synthesis:CCl4 is used as a reaction medium for a variety of chemical reactions.
Safety Considerations, Which compound listed below will dissolve in carbon tetrachloride ccl4
CCl4 is a toxic substance that can cause a variety of health problems, including liver damage, kidney damage, and cancer. It is important to take appropriate safety precautions when working with CCl4, including:
- Wearing gloves and protective clothing
- Working in a well-ventilated area
- Avoiding contact with skin and eyes
Popular Questions
What factors influence the solubility of compounds in CCL4?
The solubility of compounds in CCL4 is primarily influenced by their polarity and molecular weight. Polar compounds tend to be less soluble in CCL4, while nonpolar compounds are more soluble.
What are some practical applications of CCL4 as a solvent?
CCL4 is commonly used as a solvent in various industries, including the manufacturing of dyes, pesticides, and pharmaceuticals. It is also employed in dry cleaning and as a degreasing agent.