Hypochlorous acid and calcium hydroxide, two seemingly disparate compounds, share a remarkable versatility that has propelled them to the forefront of various industries. Hypochlorous acid, with its potent disinfectant properties, has become an indispensable tool in healthcare and sanitation, while calcium hydroxide finds widespread use in construction and water treatment.
This article delves into the fascinating world of these compounds, exploring their chemical properties, practical applications, and safety considerations.
From disinfecting wounds to purifying water, hypochlorous acid and calcium hydroxide play a crucial role in maintaining public health and infrastructure. Understanding their unique characteristics and potential hazards is essential for their safe and effective utilization.
Hypochlorous Acid and Calcium Hydroxide
Hypochlorous acid (HOCl) and calcium hydroxide (Ca(OH)2) are two inorganic compounds with distinct chemical properties and applications.
HOCl is a weak acid that is produced naturally by the human immune system as part of its defense against pathogens. It is also a potent disinfectant that is commonly used in water treatment, wound care, and food processing.
Ca(OH)2, also known as slaked lime, is a strong base that is used in a variety of industrial and construction applications. It is commonly used as a flocculant in water treatment, as a binder in cement, and as a neutralizing agent in acidic environments.
Uses of Hypochlorous Acid and Calcium Hydroxide

Hypochlorous Acid
- Disinfectant: HOCl is a powerful disinfectant that is effective against a wide range of bacteria, viruses, and fungi. It is commonly used in water treatment, wound care, and food processing.
- Wound care: HOCl is used in wound care to clean and disinfect wounds, promote healing, and prevent infection.
- Other uses: HOCl is also used in a variety of other applications, including as a bleaching agent, a deodorizer, and a preservative.
Calcium Hydroxide, Hypochlorous acid and calcium hydroxide
- Construction: Ca(OH)2 is used as a binder in cement and mortar. It is also used as a whitewash and as a plaster.
- Water treatment: Ca(OH)2 is used as a flocculant in water treatment to remove impurities and clarify water.
- Neutralizing agent: Ca(OH)2 is used as a neutralizing agent in acidic environments to raise the pH level.
- Other uses: Ca(OH)2 is also used in a variety of other applications, including as a fertilizer, as a soil amendment, and as a flux in welding.
Chemical Reactions Involving Hypochlorous Acid and Calcium Hydroxide
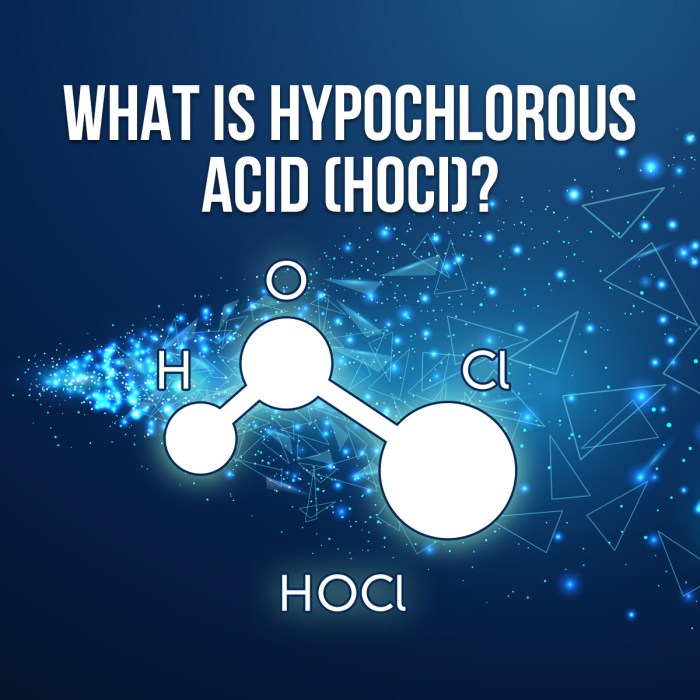
HOCl and Ca(OH)2 react to form calcium hypochlorite (Ca(OCl)2) and water (H2O).
HOCl + Ca(OH)2 → Ca(OCl)2 + 2H2O
Calcium hypochlorite is a strong oxidizing agent that is used as a disinfectant and bleaching agent.
Safety Considerations
HOCl and Ca(OH)2 are both corrosive substances that can cause skin irritation and burns. They should be handled with care and appropriate personal protective equipment (PPE) should be worn.
HOCl is a strong oxidizing agent and can react violently with organic materials. It should be stored in a cool, dark place away from flammable materials.
Ca(OH)2 is a strong base and can cause eye damage. It should be stored in a dry place away from moisture.
Comparison of Hypochlorous Acid and Calcium Hydroxide
| Property | Hypochlorous Acid | Calcium Hydroxide |
|---|---|---|
| Chemical formula | HOCl | Ca(OH)2 |
| Appearance | Clear liquid | White powder |
| pH | 2-3 | 12-13 |
| Solubility | Soluble in water | Slightly soluble in water |
| Uses | Disinfectant, wound care, bleaching agent | Construction, water treatment, neutralizing agent |
| Safety | Corrosive, oxidizing agent | Corrosive, strong base |
Case Studies or Examples
- HOCl is used in wound care to treat diabetic foot ulcers. It has been shown to be effective in reducing infection and promoting healing.
- Ca(OH)2 is used in water treatment to remove impurities and clarify water. It is also used in the production of paper and textiles.
Future Research Directions
There are a number of potential areas for future research on HOCl and Ca(OH)2.
- The development of new and more effective applications for HOCl and Ca(OH)2 in water treatment, wound care, and other fields.
- The study of the long-term effects of HOCl and Ca(OH)2 on human health and the environment.
- The development of new methods for the production and storage of HOCl and Ca(OH)2.
Continued research on HOCl and Ca(OH)2 is important to ensure that these compounds are used safely and effectively.
Expert Answers: Hypochlorous Acid And Calcium Hydroxide
What is the chemical formula for hypochlorous acid?
HOCl
What is the pH of a calcium hydroxide solution?
12.5
Is hypochlorous acid safe to use on skin?
Yes, when diluted to appropriate concentrations
What is the primary use of calcium hydroxide in construction?
As an additive to cement and mortar